Accelerated Stability Studies Pdf
For each batch of the new drug product described in the registration application the. Additional topics address assessment of product transport conditions on stability and accelerated stability testing.
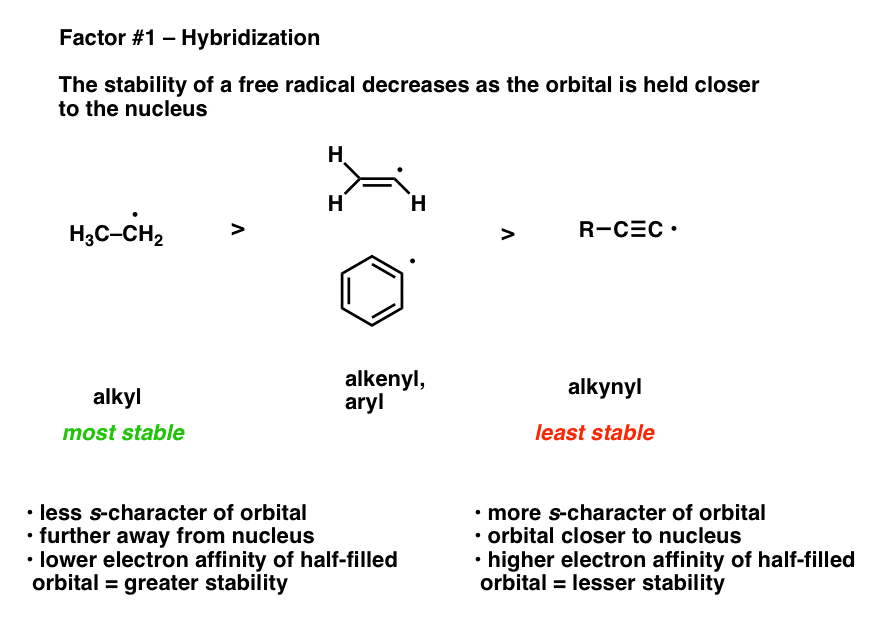
What Factors Destabilize Free Radicals Master Organic Chemistry
They find that IGF2BPs selectively bind to m6A-containing mRNAs and promote their stability.
. Requiring only commercially available materials mild conditions and operationally trivial reaction protocols we anticipate this carbon-carbon bond-forming protocol will be widely used in. ICH Q6A and Q6B should be consulted for recommendations on the setting and justification of acceptance criteria and ICH Q1D. Identify IGF2BPs as an additional class of N6-methyladenosine m6A reader proteins.
Nurses often work with multiple patients who have a variety of health needs. Significant Change in Pharmaceutical Stability Testing. Following are ICH stability conditions for these zones.
Web The good stability of PAA5 was also verified by 1 H NMR studies and UVVis spectra Supplementary Figs. The ability to coordinate numerous treatment plans and records is critical to ensure that each. Web Simulation of complex physical systems described by nonlinear partial differential equations PDEs is central to engineering and physical science with applications ranging from weather 1 2 and climate 3 4 and engineering design of vehicles or engines to wildfires and plasma physics Despite a direct link between the equations of motion and the.
Web stability studies using single- or multi-factor designs and full or reduced designs. 214 Container Closure System The stability studies should be conducted on the active substance packaged in a container closure system that is the same as or simulates the packaging proposed for storage and distribution. Web Emotional stability.
Web These stability studies zones are created due to the difference in temperature and humidity in different parts of the world. Web procedure validation studies and from long-term and accelerated stability studies should be provided. Web the design implementation data analysis and documentation needs for studies to establish and verify shelf life and in-use life of IVD reagents.
These zones have different ICH stability conditions for pharmaceutical products. Clinical and Laboratory Standards Institute CLSI. Web Huang et al.
Web accelerated testing should cover a minimum of 6 months duration at the time of submission. The mission of WHO prequalification is to work in close cooperation with national regulatory agencies and other partner organizations to make quality priority medical products available for those who urgently need them. Web World Health Organization Prequalification.
The term room temperature refers to the general customary environment and should not be inferred to be the storage statement for labeling. Web In summary the concept of accelerated serendipity has been successfully executed resulting in the discovery of a photoredox amine CH arylation reaction. The excellent stability of PAA4 and PAA5 provides a structural basis.
S9 S12 and S13. One batch with three months accelerated stability data reported in prior approval supplement and long-term stability data of first production batch reported in annual report. The applicant should ensure that complete degradation product profiles eg chromatograms of individual batches are available if requested.
Registered nurses need emotional resilience and the ability to cope with human suffering emergencies and other stressors.
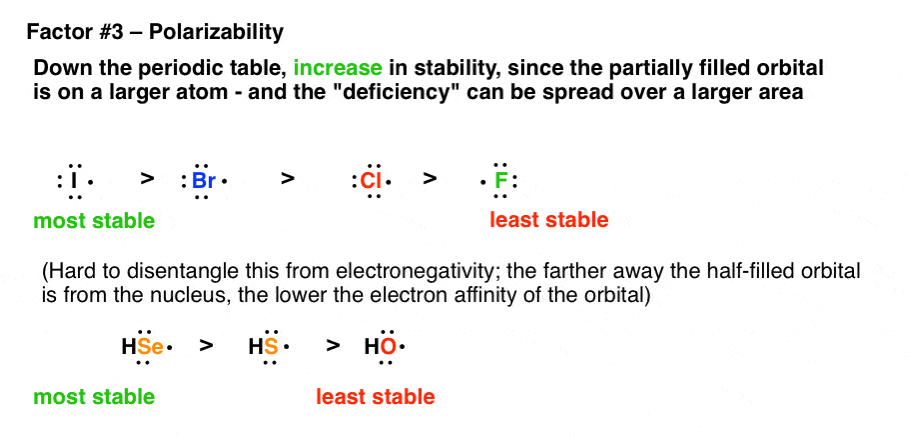
What Factors Destabilize Free Radicals Master Organic Chemistry

Physical Science Scope And Sequence It S Not Rocket Science Physical Science High School Physical Science Physical Science Experiments
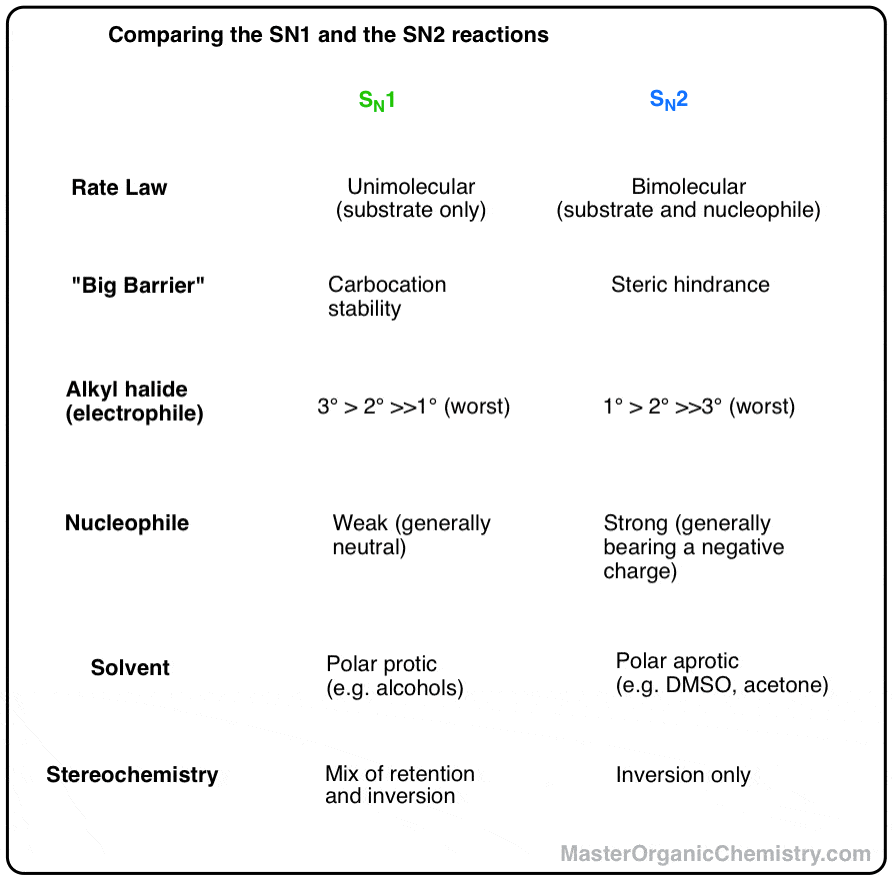
Comparing The Sn1 Vs Sn2 Reactions Master Organic Chemistry
0 Response to "Accelerated Stability Studies Pdf"
Post a Comment